Abstract
Background
Accurate measurement of disease associated with endemic bacterial agents in pig populations is challenging due to their commensal ecology, the lack of disease-specific antemortem diagnostic tests, and the polymicrobial nature of swine diagnostic cases. The main objective of this retrospective study was to estimate temporal patterns of agent detection and disease diagnosis for five endemic bacteria that can cause systemic disease in porcine tissue specimens submitted to the Iowa State University Veterinary Diagnostic Laboratory (ISU VDL) from 2017 to 2022. The study also explored the diagnostic value of specific tissue specimens for disease diagnosis, estimated the frequency of polymicrobial diagnosis, and evaluated the association between phase of pig production and disease diagnosis.
Results
S. suis and G. parasuis bronchopneumonia increased on average 6 and 4.3%, while S. suis endocarditis increased by 23% per year, respectively. M. hyorhinis and A. suis associated serositis increased yearly by 4.2 and 12.8%, respectively. A significant upward trend in M. hyorhinis arthritis cases was also observed. In contrast, M. hyosynoviae arthritis cases decreased by 33% average/year. Investigation into the diagnostic value of tissues showed that lungs were the most frequently submitted sample, However, the use of lung for systemic disease diagnosis requires caution due to the commensal nature of these agents in the respiratory system, compared to systemic sites that diagnosticians typically target. This study also explored associations between phase of production and specific diseases caused by each agent, showcasing the role of S. suis arthritis in suckling pigs, meningitis in early nursery and endocarditis in growing pigs, and the role of G. parasuis, A. suis, M. hyorhinis and M. hyosynoviae disease mainly in post-weaning phases. Finally, this study highlighted the high frequency of co-detection and -disease diagnosis with other infectious etiologies, such as PRRSV and IAV, demonstrating that to minimize the health impact of these endemic bacterial agents it is imperative to establish effective viral control programs.
Conclusions
Results from this retrospective study demonstrated significant increases in disease diagnosis for S. suis, G. parasuis, M. hyorhinis, and A. suis, and a significant decrease in detection and disease diagnosis of M. hyosynoviae. High frequencies of interactions between these endemic agents and with viral pathogens was also demonstrated. Consequently, improved control programs are needed to mitigate the adverse effect of these endemic bacterial agents on swine health and wellbeing. This includes improving diagnostic procedures, developing more effective vaccine products, fine-tuning antimicrobial approaches, and managing viral co-infections.
Introduction
Streptococcus suis (S. suis), Glaesserella parasuis (G. parasuis), Mycoplasma hyorhinis (M. hyorhinis), Actinobacillus suis (A. suis), and Mycoplasma hyosynoviae (M. hyosynoviae) are among the most significant bacterial pathogens of swine that can cause systemic disease in swine [1]. In addition to their direct impact on pig health and productivity, they are key drivers of antimicrobial use on farms [2]. While these bacteria can be commensals of the upper respiratory tract of pigs, they can also cause severe systemic disease often culminating in increased herd morbidity and mortality [3]. Colonization of piglets by these agents can occur through vertical transmission, contact with the sow during the suckling period, as well as between pigs in the nursery and postweaning stages [4,5,6].
Although colonization usually occurs early in life and is widespread in the population, various factors such as incubation period, pathogenicity of strains, polymicrobial interactions, commingling of pigs of different ages and sources, and other husbandry and management practices determine disease expression at different phases of the production cycle [3]. Thus, control programs for these agents require a multifaceted approach that enhances immunity and minimizes bacterial load through gilt acclimation (e.g., homologous sow herd exposure), management practices and pig flow, and strategic use of antimicrobials and commercial and autogenous vaccines [7,8,9].
Disease associated with S. suis and G. parasuis are commonly observed in suckling and nursery pigs (~ 10 weeks of age) with a spectrum of lesions that vary from arthritis, bronchopneumonia, meningitis, polyserositis, and acute death [10, 11]. M. hyorhinis commonly causes polyserositis and arthritis in nursery pigs between 3 and 10 weeks of age [12, 13]. Bronchopneumonia, pleuritis, polyserositis, and acute death are the most common manifestations of A. suis-associated disease, which occurs mainly in the grow-finish phase in pigs between 10 and 16 weeks of age, but can also be seen in pre-weaning piglets in high health herds [14,15,16]. Similarly, the disease associated with M. hyosynoviae is commonly observed in later phases of pig development as arthritis in pigs between 12 and 22 weeks of age [17, 18].
Accurate measurement of disease associated with endemic bacterial agents in pig populations is challenging due to their commensal ecology, the lack of disease-specific antemortem diagnostic tests, and the polymicrobial nature of swine diagnostic cases [19]. Therefore, the main objective of this retrospective study was to estimate temporal patterns of agent detection and disease diagnosis for S. suis, G. parasuis, M. hyorhinis, A. suis, and M. hyosynoviae in porcine tissue specimens submitted to the Iowa State University Veterinary Diagnostic Laboratory (ISU VDL). The study also explored the diagnostic value of specific tissue specimens for disease diagnosis, estimated the frequency of polymicrobial diagnosis and evaluated the association between phase of pig production and disease diagnosis.
Materials and methods
Study overview
The ISU VDL is fully accredited by the American Association of Veterinary Laboratory Diagnosticians according to the ISO 17025 since 2007. In 2022, the laboratory received over 88,134 porcine diagnostic cases. A diagnostic case (the unit of analysis) consists of the specimens and associated information provided by the submitting veterinarian for the investigation of a disease event on one swine farm, as well as the test results and diagnostic interpretation provided by the diagnostician. This information is stored in a customized Laboratory Information Management System (LIMS) on a standardized disease diagnosis coding system [20].
The detection of the five bacterial agents in this study was based on standard bacteriological isolation techniques, polymerase chain reaction (PCR) assays, or both. Culture of S. suis, G. parasuis, and A. suis was performed using standard procedures [21], with taxonomic identification by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF, MALDI Biotyper®, Bruker, Billerica, MA USA) [22]. Due to their fastidious nature, culture of M. hyorhinis and M. hyosynoviae is not routinely performed. For nucleic acid detection, extraction of G. parasuis, M. hyorhinis, and M. hyosynoviae DNA was performed using the MagMAX™-96 Pathogen RNA/DNA kit (Applied Biosystems™, Carlsbad, CA USA) on the automated Kingfisher™ Flex Purification System (Thermo Fisher Scientific, Inc., Waltham, MA USA) followed by amplification using specific PCRs, as described elsewhere [23, 24]. The detection information is given to the level of sample, and this information is uploaded by laboratory personnel to the ISU VDL LIMS.
For disease diagnosis, a pathological evaluation is conducted by ISU VDL diagnosticians, and diagnostic codes (DxCodes) are assigned to the case based on the interpretation of the clinical history, diagnostic testing, and pathological changes. Derscheid et al. [20] highlights that disease diagnostic codes are a summarization of the case and they then serve as a proxy for querying data for stakeholder support, surveillance, and benchmarking of animal diseases in their herds. Briefly, the DxCodes included in this study were related to body system, insult, lesion, and etiology. A summary of the terms used in this study is given in Supplementary Table 1.
The ISU VDL LIMS database search was performed from January 1, 2017, and December 31, 2022. The search was conducted for diagnostic tests (e.g., culture and/or PCR) associated with the detection of the five agents. Information gathered from the diagnostic test included the type of bacterial agents detected, the specimens and lesions associated with disease associated to these five agents. Concerning disease diagnosis, the DxCode search was conducted based on the list of codes associated with S. suis, G. parasuis, A. suis, M. hyorhinis, and M. hyosynoviae infection (Supplementary Table 2). After that, analyses of temporal trends in agent detection or disease diagnosis and relevant associations were analyzed at the case level.
Data management
The data was generated by extracting, integrating, aggregating, and summarizing information within a porcine diagnostic case [25, 26]. A diagnostic case may contain one or more pigs with different agents detected in different specimens or include one or more DxCodes. In this study, a case represented a detection or disease event in a pig population. Three modified datasets of bacteriology, PCR, and DxCode containing aggregated data by accession number were created using functions from dplyr and tidyverse packages in R (version 4.2.1, R core team 2022).
Overall, bacterial detection datasets included information on pathogen isolated or PCR results (positive, suspect and negative), case year, and specimens used for isolation or PCR testing for each case. With detection datasets, specimens that can support disease diagnosis of the five studied agents were selected and then recoded and grouped into sets. The set of specimens identified as central nervous system (CNS) samples included brain, brain swab, cerebrospinal fluid, cerebellum, spinal cord, spinal cord swab, and meninges. The serosal fibrin set included abdominal fluid, fibrin, fibrin swab, liver surface, lung surface, spleen surface, peritoneal fluid, peritoneal fluid swab, peritoneum, pleura, pleural swab, pleural fluid, heart surface, heart, heart swab epicardium, pericardium, pericardial sac, pericardial fluid, and pericardial swab. The set of heart valve includes only heart valvular leaflets. The joint set included hoof, joint, joint capsule, joint fluid, joint swab, joint carpus, joint fetlock, synovial fluid, synovial membrane, and synovial tissue. The kidney set included kidney and kidney swab. The liver set included liver and liver swab. The lung set included lung and lung swab. The spleen set included spleen. All remaining specimens were classified as other or non-identified. Thus, cases that lacked identification of specimens (e.g., cases that included “culture”, “assorted” or “tissue” as reported specimens) were also not considered in the analyses of detection trends. To count number of cases with a given specimen each year, a new variable “agent + specimen” was created by concatenating the column including pathogen identification and specimen for detection. After that, data were aggregated by year and duplicated accession identifiers were removed, and then counts and trend analyzes were performed using the variable agent + specimen, as described in the statistical methods.
The DxCode dataset included variables of animal identification, age, etiology identified, affected body system (cardiovascular, musculoskeletal, nervous, respiratory, and systemic), lesion (arthritis, bronchopneumonia, endocarditis, meningitis, serositis, and sepsis) for each animal in each case. Each case could have included one or a combination of lesions. Research, ancillary and cases that included non-infectious etiology and non-pathological lesions (e.g., anomalies, metabolic, traumas, etc.) were removed. Categorization of case age of pigs into production phase was carried out as follows: 1) suckling piglets (0 < x ≤ 3-week-old); 2) early-nursery (3 < x ≤ 6-week-old); 3) late-nursery (6 < x ≤ 10-week-old); 4) growing (10 < x ≤ 16-week-old); and 5) finishers/adults (16-week-old < x). Then, a new variable was created, namely “etiology + lesion”, where infectious etiology and lesion Dx Codes were concatenated. To count the number of cases for each agent associated with a lesion each year, the matching pair of etiology + lesion variable was aggregated by year and duplicated accession identifiers were removed, and then count and trend analyses were performed, as described in the statistical methods.
Data analyses
Trend analyses by agent
The detection rates by year for culture (S. suis, G. parasuis, and A. suis) and PCR (G. parasuis, M. hyorhinis, and M. hyosynoviae) were estimated using binomial regression models. The proportion between number of cases with at least one bacterial isolation or positive PCR result in at least one specimen of interest for each agent divided by total culture or total PCR testing was considered the dependent variable and year the independent variable.
Likewise, disease diagnosis rates per year for each agent by a compatible lesion were calculated using binomial regression models. S. suis disease diagnosis trends were estimated for arthritis, bronchopneumonia, endocarditis, meningoencephalitis, sepsis, or serositis cases; G. parasuis disease diagnosis trends were estimated for arthritis, bronchopneumonia, meningoencephalitis, sepsis, or serositis; M. hyorhinis disease diagnosis trends were estimated for arthritis, bronchopneumonia, or serositis; A. suis disease diagnosis trends were estimated for arthritis, bronchopneumonia, sepsis, or serositis; and trends for M. hyosynoviae disease were estimated for arthritis. For all these models, the number of disease diagnosis cases for each agent associated with a specific lesion was divided by the total porcine diagnostic cases with a related assigned infectious etiology with the associated lesion by each year (Supplementary Table 3) and was considered the dependent variable, and the year was the independent variable.
Relationship between disease diagnosis and specimens of detection
This evaluation aimed to contrast pathogen detection and disease diagnosis by evaluating the detection of target pathogens in specimens of interest and an established disease diagnosis (etiology associated with a lesion). The numerator represented the total number of disease cases with an etiology and associated lesion (etiology + lesion), and the denominator included cases that included detection for the given agent along with a specific specimen (agent + specimen) but also included a histopathology assessment to guarantee that the case was also evaluated for disease diagnosis. This strategy was chosen due to the absence of specimen information in the pathology dataset and that not all specimens were evaluated for lesions. To illustrate the detection of S. suis in CNS samples with S. suis meningitis disease, the number of cases of S. suis meningitis was divided by the number of cases that simultaneously included S. suis detection in CNS samples and DxCodes for any disease.
Disease diagnosis by production phase and agent
Comparisons of number of cases by production phase was done using Poisson regressions for each agent within each lesion.
Polymicrobial diagnosis
Polymicrobial diagnosis in this study were related to the presence of disease caused by more than one infectious etiology, for example, a DxCode including PRRSV and S. suis. The frequencies of polymicrobial diagnosis among the five agents and other porcine pathogens were analyzed using the body systems (cardiovascular, musculoskeletal, nervous, respiratory, and/or systemic). Cases that were given more than two body system DxCodes (e.g., respiratory + cardiovascular; nervous + systemic), i.e., more than one body system was involved in the whole case, were all classified as multisystemic to facilitate interpretation.
Results
Bacterial culture frequencies and detection trends
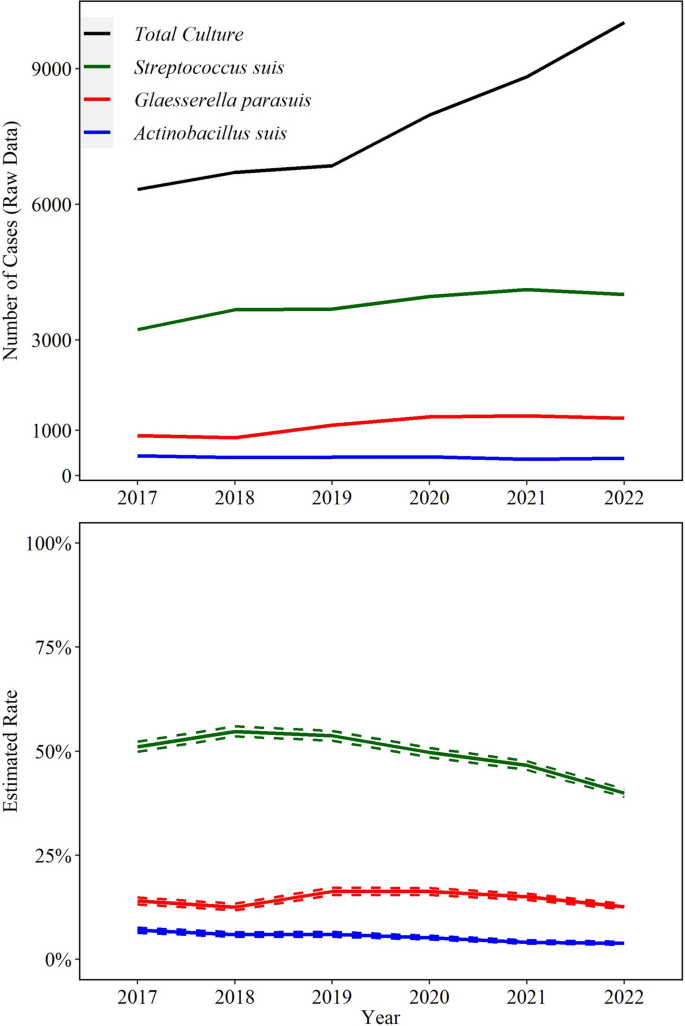
A total of 82,563 cases from 2017 to 2022 identified at least one bacterial agent relevant to swine health in any specimen. From those, 26,889 (33%) included isolation of one or more of the three bacterial agents detected by culture included in this study; S. suis (22,675; 27%), G. parasuis (6753; 8%), and A. suis (2429; 3%) were isolated from any specimen. Of these, S. suis was isolated from specimens of interest (CNS, heart valve, joint, kidney, liver, lung, serosal fibrin, spleen) in 21,190 (93%) cases, G. parasuis was isolated from these specimens in 6612 (98%) cases, and A. suis isolation in 2344 (97%) cases Fig 1.
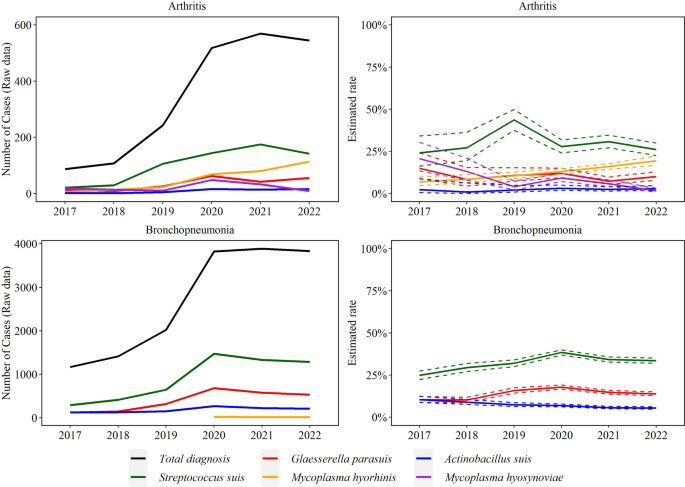
Trendlines (raw data and estimated rate) of overall detection of Streptococcus suis, Glaesserella parasuis, and Actinobacillus suis using bacteriologic culture. The annual estimated rate (solid line) and 95% confidence interval (dashed line) referred to the output of a Binomial regression model which modeled the total number of cases with detection of each agent (colored lines in raw data plot) divided by the total number of bacterial cultures for each year (black line in raw data plot). Black line is based on specimens of interest (see Fig. 2)
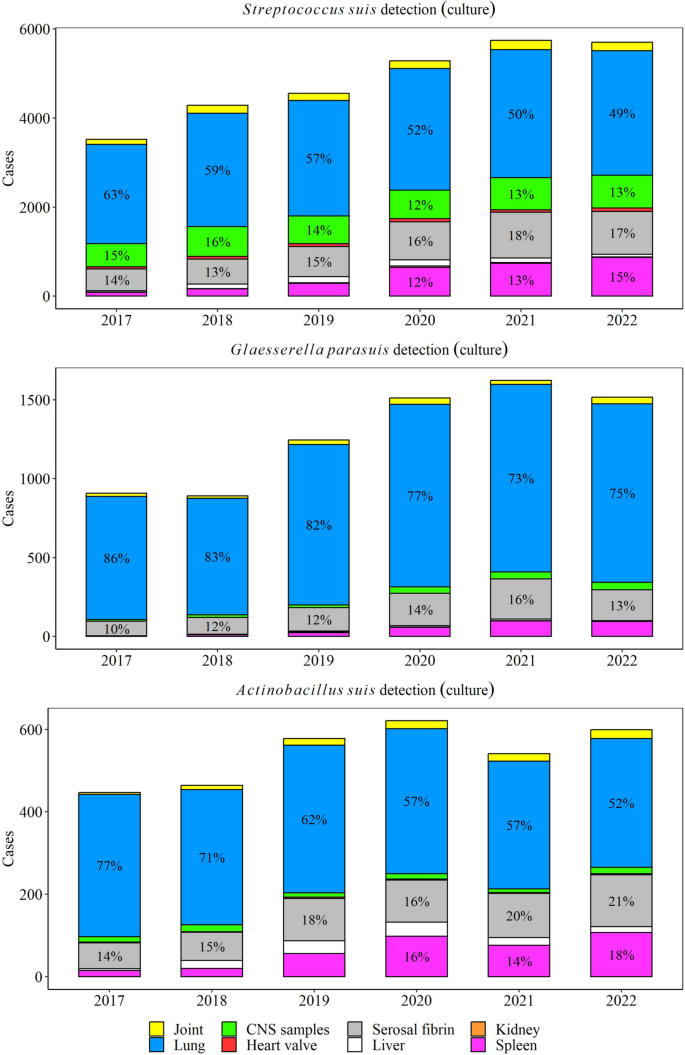
Lungs (50% yearly), serosal fibrin (17% yearly), and CNS (13% yearly) were the most common specimens for S. suis isolation, while lungs (75% yearly) and serosal fibrin (13% yearly) were the main specimens for G. parasuis isolation. A. suis was mostly isolated from lungs (52% yearly), serosal fibrin (20% yearly), and spleen (17% yearly) (Fig. 2).
While the bacterial culture rate of S. suis peaked in 2018, this decreased by an average of 9.9% (95% CI 8.9, 11.0%) in the later years (2019–2022, P ≤ 0.05). Culture of A. suis decreased significantly by an average 12.0% (95% CI 9.7, 14.0%, P ≤ 0.05) per year. G. parasuis cultures peaked in 2019, but overall the rate did not change in the study period (P ≤ 0.05, Fig. 1).
PCR detection frequencies and trends
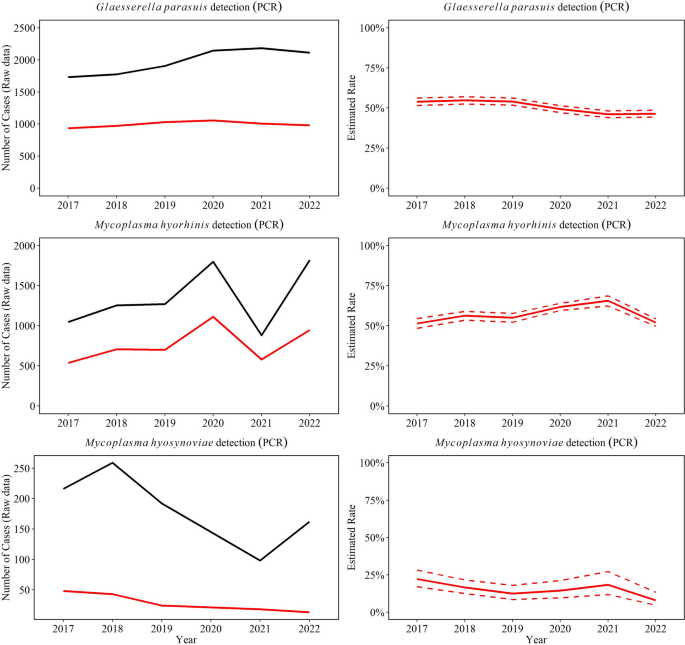
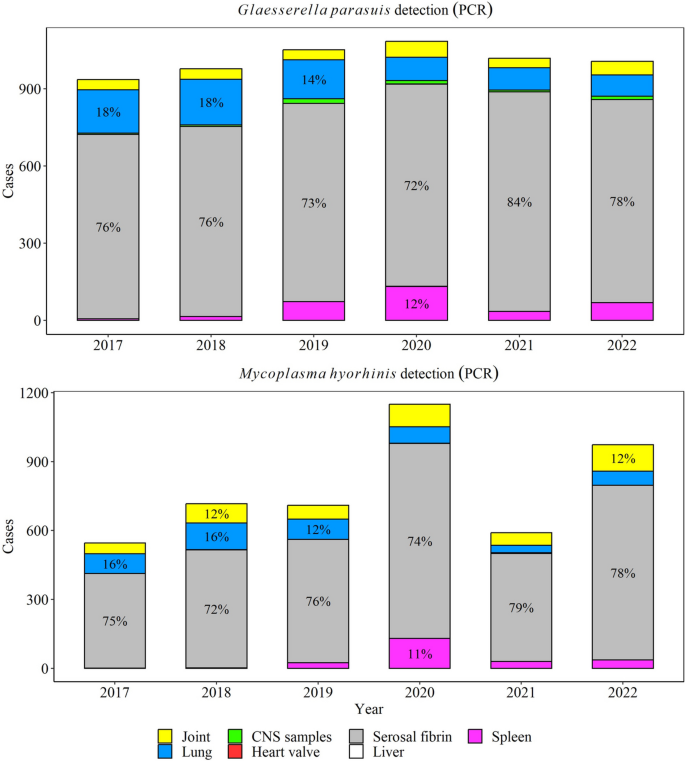
Between 2017 through 2022, a total of 50% (5981/11,849) G. parasuis PCR tests were positive in specimens of interest (CNS, heart, serosal fibrin, joint, kidney, liver, lung, and spleen). Overall, there was a significant decrease in the rate of positive PCR results for G. parasuis by 0.7% (95% CI 0.5, 0.9%) over the study period (P ≤ 0.05, Fig. 3), and the highest detection was observed in 2018. Among G. parasuis PCR-positive tests, serosal fibrin (77% yearly) and lungs (12% yearly) were the most common specimens for G. parasuis detection (Fig. 4).
Trendlines (raw data and estimated rate) of overall detection of Glaesserella parasuis, Mycoplasma hyorhinis, and Mycoplasma hyosynoviae using PCR testing in specimens to diagnose disease. The red line represents the positive test results and black line the total testing. The annual estimated rate (solid line) and 95% confidence interval (dashed line) referred to the output of a Binomial regression model which modeled the total number of PCR-positive results from each agent (red line in raw data plot) divided by the total number of PCR tests from each agent for each year (black line in raw data plot)
M. hyorhinis was detected in 57% of all M. hyorhinis PCR tests performed (4578 /8069), with serosal fibrin (75% yearly) and joints (10% yearly) being the main specimens tested (Fig. 4). M. hyorhinis PCR positivity rate peaked in 2021 (P ≤ 0.05, Fig. 3), but overall, it remained steady over the study period (P > 0.05).
M. hyosynoviae was detected in joints in 22% of all M. hyosynoviae PCR tests cases (221/1006) and decreased significantly by an average of 16.0% (95% CI 6.4, 25.0%) per year (P ≤ 0.05, Fig. 3).
Disease diagnosis frequencies
Based on pathologic assessment over 6 years, 44,731 swine cases rendered a disease diagnosis with at least one DxCode with a viral or bacterial infectious etiology. From those, 15,990 (35.7%) cases included disease diagnosis of at least one of the five studied agents. From those cases, 61.7% were S. suis (n = 9862), 36.0% G. parasuis (n = 5760), 21.0% M. hyorhinis (n = 3349), 9.5% A. suis (n = 1520), and 0.8% M. hyosynoviae (n = 133). The frequency of these five agents related to the total lesions under disease diagnosis observed in the ISU VDL is shown in Table 1. The annual frequency of lesions associated with systemic infection is shown in Fig. 5. M. hyosynoviae was only associated with arthritis cases.
Disease diagnosis trends
There was a significant increase in disease diagnosis for all agents over the study period, except for M. hyosynoviae, (Figs. 6–8). Specifically, the rate of M. hyorhinis detection in arthritis cases (average 51 cases/year) increased consistently by 26.8% (95% CI 14.9, 40.0%) average yearly for the studied period (P ≤ 0.05). In contrast, the rate of M. hyosynoviae arthritis (22 cases/year) decreased by 33.0% (95% CI 24.0, 40.0%) on average each year (P ≤ 0.05). S. suis arthritis peaked in 2019 but overall, no significant trend was detected for S. suis (103 cases/year), G. parasuis (35 cases/year), or A. suis (9 cases/year) in arthritis cases (P > 0.05).
Trendlines (raw data and estimated rate) of arthritis and bronchopneumonia observed with Streptococcus suis, Glaesserella parasuis, Mycoplasma hyorhinis, Actinobacillus suis, and Mycoplasma hyosynoviae disease diagnoses. The annual estimated rate (solid line) and 95% confidence interval (dashed line) referred to the output of a binomial regression model which modeled the total number of cases of each agent (colored lines in raw data plot) divided by the total number of cases of the specific lesion for each year (black line in raw data plot)
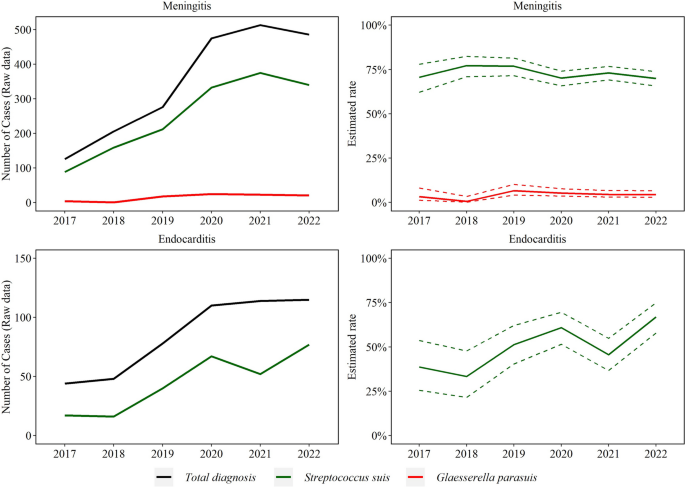
Trendlines (raw data and estimated rate) of endocarditis and meningitis observed with Streptococcus suis and Glaesserella parasuis disease diagnosis. The annual estimated rate referred to the output of a binomial regression model in which modeled the total number of cases of each agent (colored lines in raw data plot) divided by the total number of cases of the specific lesion for each year (black line in raw data plot)
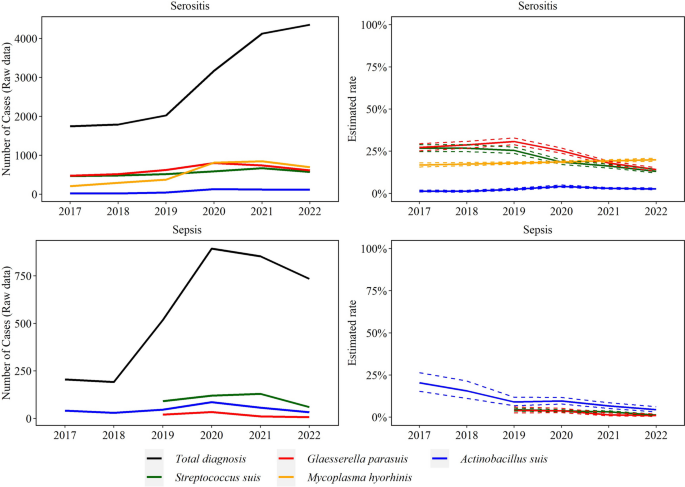
Trendlines (raw data and estimated rate) of sepsis and serositis observed with Streptococcus suis, Glaesserella parasuis, Mycoplasma hyorhinis, Actinobacillus suis, and Mycoplasma hyosynoviae disease diagnoses. The annual estimated rate (solid line) and 95% confidence interval (dashed line) referred to the output of a binomial regression model which modeled the total number of cases of each agent (colored lines in raw data plot) divided by the total number of cases of the specific lesion for each year (black line in raw data plot)
The rate of S. suis (907 cases/year) and G. parasuis (397 cases/year) bronchopneumonia cases increased respectively by 6.0% (95% CI 3.7, 8.3%) and 4.3% (95% CI 0.2, 7.5%) on average each year (P ≤ 0.05). In contrast, A. suis bronchopneumonia (184 cases/year) decreased by 13.2% on average each year (95% CI 9.8, 16.5%) (P ≤ 0.05). A total of 23, 13, and 15 cases of M. hyorhinis bronchopneumonia were observed in 2020, 2021, and 2022, respectively.
No significant trend was detected in S. suis (252 cases/year) and G. parasuis (n = 16 cases/year) meningitis (P > 0.05). S. suis endocarditis (45 cases/year) increased by 23.0% (95% CI 9.8, 38.0%) on average each year (P ≤ 0.05, Fig. 7). Trend of A. suis endocarditis (4 cases total) and meningitis (15 cases over the study period) were not estimated because of the small sample size.
S. suis (101 cases/year), G. parasuis (18 cases/year), and A. suis (50 cases/year) sepsis cases decreased over the study period by 30.0% (95% CI 23.0, 36.0%), 41.0% (95% CI 26.0, 54.0%), and 27.0% (95% CI 21.0, 32.0%), respectively (P ≤ 0.05).
The rate of M. hyorhinis (n = 539 cases/year) and A. suis (78 cases/year) serositis cases increased by 4.2% (95% CI 1.7, 6.7%) and 12.9% (95% CI 6.4, 19.9%), respectively. S. suis (549 cases year) and G. parasuis (630 cases/year) cases decreased by an average of 18.0% (95% CI 16.0, 20.0%) and 17.0% (95% CI 16.0, 19.0%) in each year, respectively (P ≤ 0.05, Fig. 8).
Relationship between disease diagnosis and specimens
The denominators for this analysis were cases that included agent detection in specific specimen and histopathology assessment using any specimen. The frequencies of specimens used to assess lesions given that the agent was isolated or detected by PCR are shown in Tables 2 and 3, respectively. For S. suis, 72% of isolates from heart valve were given a final diagnosis of S. suis endocarditis, 62.1% of isolates from joints were given a final diagnosis of S. suis arthritis and 40.3% of isolates from lungs were given a final diagnosis of S. suis bronchopneumonia.
For G. parasuis, 87.4% of isolates and 72.5% of PCR detections from serosal fibrin (such as fibrin, liver surface, lung surface, spleen surface, pleura, heart, etc.) were associated with a final diagnosis of G. parasuis serositis, while 42.3% of G. parasuis isolates and 22.7% of PCR detections from the lung were associated with bronchopneumonia. Approximately 41% of G. parasuis isolates from CNS specimens were given a final diagnosis of G. parasuis meningitis. Similarly, 42.5% of G. parasuis PCR-positive CNS specimens were given a final diagnosis of G. parasuis meningitis.
For A. suis, 77.6 and 60.9% of isolates recovered from joints and lungs were given a final diagnosis of A. suis arthritis and bronchopneumonia, respectively. Like G. parasuis, 73.4% of M. hyorhinis PCR-positive serosal fibrin tests were given a final diagnosis of M. hyorhinis serositis. Finally, 83.1% of M. hyosynoviae PCR-positive joint samples were diagnosed as M. hyosynoviae arthritis.
Association between pig production phase and specific agent-associated lesions
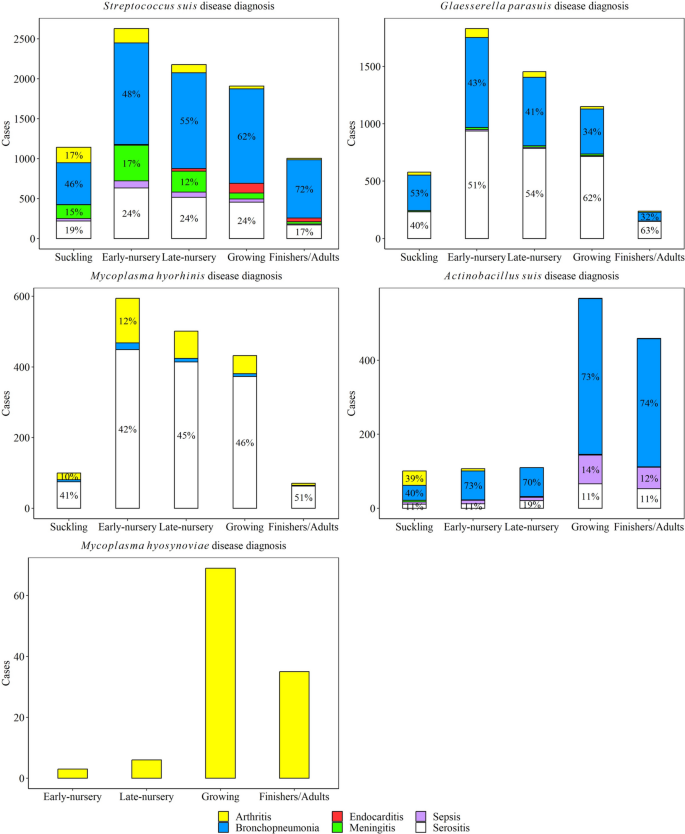
The distribution of pig production phase by lesion for all five agents is shown in Fig. 9. S. suis arthritis cases were higher in suckling and early-nursery pigs, while S. suis bronchopneumonia disease was higher in early-, late-nursery, and growing pigs (P ≤ 0.05). S. suis endocarditis was highest in the growing phase, while S. suis meningitis was highest in early-nursery compared to all other phases (P ≤ 0.05). Sepsis and serositis cases due to S. suis were statistically higher in early-nursery, late-nursery, and growing pigs than in the suckling and finishing pigs (P ≤ 0.05).
Distribution of lesions observed with Streptococcus suis, Glaesserella parasuis, Mycoplasma hyorhinis, Actinobacillus suis, and Mycoplasma hyosynoviae diseases by age of pigs. Suckling piglets (0 < x ≤ 3-week-old); early-nursery (3 < x ≤ 6-week-old); late-nursery (6 < x ≤ 10-week-old); growing (10 < x ≤ 16-week-old); and finishers and adults (16-week-old < x). The percent are shown with pig production phases that were included > 10% of cases
G. parasuis arthritis and bronchopneumonia cases were statistically higher in early- and late-nursery compared to other phases (P ≤ 0.05). G. parasuis meningitis was higher in suckling, early-, and late-nursery pigs than in growing and finishing pigs (P ≤ 0.05). No statistical difference was observed in G. parasuis sepsis cases among pig production phases (P > 0.05). G. parasuis serositis cases were higher in early-nursery, compared to late-nursery and growing phases (P ≤ 0.05).
M. hyorhinis arthritis and serositis were higher in early-, late-nursery, and growing phases (P ≤ 0.05). A. suis arthritis cases were statistically higher in suckling pigs, while bronchopneumonia, sepsis, and serositis were all statistically higher in growing and finishing pigs compared to other phases (P ≤ 0.05). M. hyosynoviae arthritis cases were higher in finishing and adult pigs (P ≤ 0.05).
Polymicrobial diagnosis
As shown in Table 4, disease diagnoses of all five agents were most frequently observed with other infectious etiologies. The most common viral agent co-detected with disease caused by S. suis, G. parasuis, A. suis and M. hyorhinis was PRRSV, followed by IAV. When combined with other infectious etiologies, S. suis, G. parasuis, M. hyorhinis, and A. suis disease diagnoses were substantially higher compared to diagnosis on their own. M. hyosynoviae cases were more frequently diagnosed without any other etiology.
Discussion
The impact of S. suis, G. parasuis, M. hyorhinis, A. suis, and M. hyosynoviae is widespread throughout the swine production chain, resulting in detrimental effects on pig health and welfare, decreased productivity, and increased production costs. Disease control measures currently involve enhancing immunity through gilt acclimation or vaccination, mitigating known management and environmental risk factors, and implementing judicious antimicrobial use. However, due to the global decline in prophylactic and metaphylactic use of antimicrobials in the swine industry, production systems must refine prevention strategies to reduce their dependence on antimicrobials. Therefore, it becomes crucial to accurately and promptly diagnose these complex endemic agents to gain valuable insights into disease occurrence and presentation. To date, temporal detection and disease diagnosis data have been scarcely described in the literature for these five endemic bacteria. Trevisan et al. [26] described the value of robust and standardized datasets, containing diagnostic data stored at U.S. VDLs, and how they can be applied to understand PRRSV occurrence at the regional level. Thus, this study aimed to determine temporal diagnostic trends of five endemic bacterial agents from field cases using 6-years of data stored at the ISU VDL.
Establishing the disease occurrence of these endemic agents at the herd level can be problematic because of their commensal ecology, concurrent infections, and broad spectrum of clinical signs and lesions [3, 19]. Their commensal status in the respiratory tract precludes using antemortem specimens (e.g., nasal swab, oral fluid, tracheal wash, bronchiolar lavage fluid) to solely determine causation. Furthermore, detecting these agents in upper respiratory tract samples (e.g., tonsil, nasal or tracheal samples) does not necessarily imply disease. These sample types are more commonly employed in agent surveillance and disease monitoring for viral and some bacterial agents [27]. Thus, disease diagnosis often requires isolation or detection in specific tissues with compatible lesions. This study used field cases containing a predefined set of specimens where these agents are more likely to be related to the clinical outcome.
There was an overall numerical and sometimes statistical increase in the detection and disease diagnosis rates for all agents in the last 6 years, except for M. hyosynoviae. The reason for this increase is likely multifactorial. From a data analysis perspective, the findings may reflect the recent optimization in data management in the ISU VDL. For example, ISU VDL improved the standards for DxCode in 2019 with changes in the DxCode format, setting a new standard and clarification of diagnostic coding among the diagnosticians [20, 25]. This change could have a role in the increase in the number of some disease diagnoses, such as bronchopneumonia, from 2019 to 2022 observed in this study. From an industry perspective, the growth of the swine industry observed between 2015 and 2019 (550,000 more sows that were younger and less-immune were added to the U.S. breeding herd inventory) [28], and/or industry-wide management changes (e.g., reduction in antimicrobial use) [29, 30] could have contributed to the noted increase in the sample submission and disease diagnosis. From a surveillance, disease management, and diagnostic perspective, the increase could also be due to amplified awareness of the impact of these agents and the advancements in diagnostic techniques, e.g., novel PCRs and whole genome sequencing implemented in diagnostic laboratories [7, 31]. The increased number of cases including a specific sample type (e.g., spleens) for agent detection might be due to the collection of that sample type for African Swine Fever and Classical Swine Fever surveillance purposes [32], thus resulting in the increased detection and disease diagnosis of these bacterial agents in recent years.
The increase could also reflect a growing interest by the swine industry in more in-depth testing, such as serotyping, whole genome sequencing, antimicrobial susceptibility testing, and the need for bacterial isolates for autogenous vaccine production. Finally, fluctuations in agent detection trends might also be due to changes in the prevalence of the agent or the dissemination of more pathogenic variants. S. suis sequence type 1 strains, a genotype considered of high pathogenicity and previously thought to be mainly found in European and Asian countries, has been more often detected in U.S. swine herds [33]. Similarly, serotype 7 G. parasuis strains, previously considered non-pathogenic, have recently been described as the most frequently detected genotype associated with disease [34, 35]. Outbreaks of viral diseases, commonly recognized as catalysts for endemic bacterial diseases, exemplified by the PRRSV-2 variant within lineage 1C diseases, could have also contributed to the observed increase [36]. Still, the precise causes of the observed increases or decreases need further elucidation.
Among the five studied agents, S. suis was the most frequently detected and diagnosed agent. In fact, 22% of all cases with infectious etiology (bacteria or viruses) included a S. suis disease diagnosis, and 27% of all bacteriology cases included S. suis isolation. Furthermore, although a trend was not detected, it was the predominant pathogen in neurological cases submitted to the ISU VDL (70.6% of all cases). Within nervous cases, S. suis disease was more frequently diagnosed without other infectious etiologies. S. suis diagnosis was regularly diagnosed in suckling and nursery phases of pig production, regardless of the lesion, as reported previously [37]. Results of this study also show that nearly half of the cases with a S. suis diagnosis occurred in pigs in the suckling, growing or finisher/adult stages, highlighting the impact of this agent across all pig production phases. The number of cases of S. suis meningitis disease was higher in suckling and early-nursery (up to 6-week-old) than late-nursery pigs, correlating with previous reports [38, 39]. This study also highlighted the significant upward trend in S. suis endocarditis in growing and finisher pig cases. Taken together, these findings highlight the significant role S. suis plays in meningitis, arthritis and serositis in younger pigs as a primary pathogen, and endocarditis in older pigs.
These data also showed constant upward trend in S. suis bronchopneumonia cases in the last 6 years; however, within bronchopneumonia cases, S. suis was co-detected with other agents, such as G. parasuis, M. hyorhinis, IAV, and PRRSV. Therefore, the significant increase of S. suis bronchopneumonia might also by explained by the increase of primary viruses (IAV and PRRSV). These observations align with previous reports on the role of S. suis as a secondary pathogen in respiratory disease cases [10]. This study demonstrated that S. suis played a role within multi systemic cases along with other infectious pathogens, e.g., S. suis disease cases were often found as unique diagnosed etiology and interacting with primary viruses and other bacteria.
G. parasuis was the second most frequently detected and diagnosed agent and one of the most diagnosed bacterial agents in serositis cases (21.9% of all cases) over the study period. It is also diagnosed in 10% of all infectious arthritis cases. Trends analyses revealed that G. parasuis serositis increased from 2017 until 2020, followed by a downward trend between 2020 and 2022. Similarly, to S. suis, G. parasuis bronchopneumonia increased significantly in the last 6 years, which could reflect the simultaneous increasing diagnosis of other etiologies, such as PRRSV. Co-diagnosis of G. parasuis with other infectious etiologies, such as PRRSV, IAV, and M. hyorhinis, was more frequent in respiratory and systemic cases (Table 4). Finally, G. parasuis disease was diagnosed mostly in the nursery phase of production, which has been previously reported [11, 40]. Thus, given the frequency of G. parasuis in nursery polymicrobial systemic disease cases, it implies that G. parasuis control likely hinges on the management of other bacterial and viral co-infections.
Trends analyses showed increased M. hyorhinis detection, mainly from 2018 through 2020, and a significant increase in M. hyorhinis serositis and arthritis cases over the study period. Specifically, 19% of all serositis and 14% of all arthritis cases were given a M. hyorhinis diagnosis (Table 1). Interestingly, Table 4 shows that M. hyorhinis disease was more commonly co-diagnosed with S. suis, G. parasuis, and PRRSV as previously reported [41,42,43]. In fact, only 8.5% of cases with an M. hyorhinis diagnosis, included only this agent. In the cases of arthritis, M. hyorhinis was more commonly diagnosed on its own (Table 4). While no significant trend was observed, there was a numerical increase in M. hyorhinis bronchopneumonia in the last 3 years. In fact, in situ hybridization with RNAscope® has recently been used to identify M. hyorhinis within lesions that were commonly associated with M. hyopneumoniae [44]. Still, the role of M. hyorhinis in this lesion is poorly understood [43]. M. hyorhinis disease was mostly diagnosed in nursery and growing pigs highlighting this agent’s the relevance in post-weaning systemic disease [45].
Disease associated with A. suis is mainly observed in cases of septicemia in suckling or recently weaned pigs, or in grow-finish pigs experiencing bronchopneumonia and serositis [6]. The similarities in clinical presentation with A. pleuropneumoniae disease complicates accurate A. suis diagnosis [46, 47]. In this study, A. suis detection and disease diagnosis were less frequent compared to S. suis, G. parasuis and M. hyorhinis. However, there was a significant increase in cases of A. suis serositis over the study period, aligning with recent reports [48]. Furthermore, A. suis was diagnosed in 8.7% of all sepsis cases, mainly in growing and finishing pigs. In contrast, a decreasing trend of A. suis bronchopneumonia and arthritis was observed which might indicate suboptimal surveillance efforts for this pathogen or simply reflect its opportunistic nature. Additionally, cases identifying A. suis arthritis were mainly seen in suckling piglets 2021 (n = 13), 2020 (n = 12 cases), and 2019 (n = 2) (Fig. 9), and not in later stages of production, suggesting that A. suis is an agent to consider in pre-weaning polyarthritis. A. suis disease diagnosis including bronchopneumonia and systemic infections were statistically associated with later phases of production in this study, correlating with previous knowledge [48]. Finally, A. suis disease as the sole etiology represented 37% of all cases with an A. suis diagnosis, suggesting a primary role in swine disease.
Regardless of the overall increase in arthritis cases observed during the study period (Fig. 6), M. hyosynoviae arthritis diagnosis significantly decreased over time. Notably, the number of cases with PCR testing for M. hyosynoviae also decreased (Fig. 4). M. hyosynoviae infection was found more often in finisher and adult pigs, as reported previously [18, 49, 50]. While data from this study show that M. hyosynoviae diagnosis decreased over the study period, reports from the field have shown that infectious lameness is a common occurrence in grow-finish herds and may play a role in sow mortality [51, 52]. The reason for the decline in diagnosis of M. hyosynoviae arthritis might be due to a decline in the submission of arthritis cases in adult pigs, since M. hyosynoviae impacts later stages of production and can be controlled via the use of antimicrobials, or it could reflect the complexity of diagnosing this agent under field settings [53]. Though there are many non-infectious and infectious causes of lameness, future epidemiology studies that include data from multiple laboratories are needed to understand the frequency of M. hyosynoviae lameness in the field.
Overall, tissues and specimens used to detect and diagnose disease of each of the five agents were submitted in accordance with their previously described pathology [19, 54, 55]. S. suis, G. parasuis, and A. suis were mainly detected through bacteriological culture using lung samples. However, from 2020 other systemic tissues were more frequently used for detection, such as CNS samples (i.e., brain, brain swab, cerebrospinal fluid), serosal fibrin, and spleen. Given that the lung may harbor a wide range of commensal bacteria [54, 56], it is critical that the diagnosis of disease is based not solely on detection data but also in association with the pathological changes. Thus, this study provided insights into which specimens were most used for disease diagnosis based on the histopathology evaluation with agent detection data. For instance, 69.6% (15,786/22,675) of all S. suis isolates originated from lungs. However, 40.3% of those lung isolates were used to diagnose S. suis bronchopneumonia cases. Only 4% of all S. suis isolates originated from joints (1025/22,675), but from those, 62.1% were given S. suis arthritis disease diagnosis. Similarly in neurological cases, 17.2% (3899/22,675) of S. suis isolates originated from CNS samples, and 48.1% of those were used to diagnose meningoencephalitis. These data imply that a significant proportion of isolates (52%) obtained from CNS samples originate from cases that, either do not have enough evidence for diagnosis (i.e., tissues were not submitted, only meningeal swabs or cerebrospinal fluid), or histopathological lesions were not observed, indicating potential cross-contamination of the sample. These data highlight the importance of proper animal selection, sample collection and handling for disease diagnosis [19]. Overall, isolation from lungs was less predictive of S. suis disease diagnosis compared to joints, serosal fibrin, and heart valves. Similar findings were observed with G. parasuis. While 89% (6015/6753) of all G. parasuis isolates were obtained from the lung, 42.3% were used to diagnose G. parasuis bronchopneumonia. Around 15% of all G. parasuis isolates were obtained from serosal fibrin samples, but from those isolations, 87.4% were used to give a G. parasuis serositis diagnosis, highlighting the diagnostic value of serosal fibrin for G. parasuis diagnosis. Assessment of clinical history, gross and microscopic changes, and isolation or detection by PCR in specific sites (CNS samples, joints, serosal fibrin, and spleen), coupled with additional diagnostic tests (serotyping and sequencing), are critical for accurate assessment of S. suis- and G. parasuis-associated diseases. Great care should be taken when collecting tissues for diagnostic submission, as S. suis or any other of the four bacterial agents are commensals that can easily confound culture or PCR results by contaminating lesioned tissues [57].
Like other studies based on diagnostic data [25, 27], caution is needed when interpreting the findings from this study, which used three large datasets created based on specific information from diagnostic cases stored in a data warehouse at a single diagnostic laboratory. Therefore, results from either detection and disease diagnosis depended on what was submitted (case and selection bias) and what was targeted by the submitter (cognitive biases) but also what information was defined as relevant by the group of diagnosticians from the ISU VDL. For instance, while these five agents can be considered secondary pathogens in the infectious process, they can also contribute to some of the observed lesions with or without other risk factors (infections/noninfectious). Thus, the DxCodes used in this study suggested what the cause of the lesion is but may not necessarily reflect main problem in the clinical context.
Additionally, true prevalence and incidence of the five agents cannot be assessed given that there is no randomness in sample collection or presence of true negative cases. Yet, this study data suggested a consistent increase in efforts to detect and diagnose diseases associated with the five endemic bacterial agents investigated and improved disease diagnostic codes. Furthermore, implementation of veterinary diagnostic codes for disease diagnosis monitoring is a recent achievement at the ISU VDL [20].
Conclusions
Understanding diagnostic trends for the primary drivers of antimicrobial use in swine farms is crucial, considering the swine industry’s emphasis on antimicrobial stewardship. This retrospective study utilized a comprehensive dataset (i.e., ~ 27 k bacteriological cases and ~ 16 k histopathological reports) collected over a 6-year period. Results from this study demonstrated significant increases in disease diagnosis for S. suis, G. parasuis, M. hyorhinis, and A. suis, and a significant decrease in detection and disease diagnosis of M. hyosynoviae. Investigation into the diagnostic value of particular tissues showed that lungs were frequently included for detection and disease diagnoses. However, the use of lung for systemic disease diagnosis requires caution due to the commensal nature of these agents in the respiratory system, compared to systemic sites that diagnosticians typically target. Bacterial isolation and DNA in systemic sites did not necessarily indicate the presence of disease caused by these five agents. For instance, half of S. suis isolation in CNS samples was used in meningitis diagnoses associated with S. suis infections. Thus, the anatomic location selected for sampling from acutely affected, nonmedicated animals, based on a case definition and proper diagnostic testing, gross lesions, and verified compatible histopathologic lesions, offers a comprehensive assessment for the contribution of these bacteria to systemic disease. Given that commercial vaccines are not available for the majority of these pathogens, it is essential to conduct a thorough diagnostic process to improve the likelihood of obtaining disease-associate strain candidates for the development of autogenous vaccines.
This study also explored associations between phase of production and specific diseases caused by each agent, showcasing the role of S. suis arthritis in suckling pigs, meningitis in early nursery and endocarditis in growing pigs, and the role of G. parasuis, A. suis, M. hyorhinis and M. hyosynoviae disease mainly in post-weaning phases. Hence, it is necessary to take into consideration the onset of disease throughout the various phases of pig production when formulating control strategies for these four agents. Finally, this study highlighted the high frequency of co-detection with other infectious etiologies, such as PRRSV and IAV, demonstrating that to minimize the health impact of these endemic bacterial agents it is imperative to establish effective viral control programs.
Availability of data and materials
The datasets presented in this article are not readily available because Client confidentiality is strictly maintained, but the data can be searched and recovered for analysis by authorized personnel only. Requests to access the datasets should be directed to Maria Jose Clavijo, mclavijo@iastate.edu.
Abbreviations
- ISU VDL:
- Iowa State University Veterinary Diagnostic Laboratory
- LIMS:
- Laboratory Information Management System
- PCR:
- Polymerase chain reaction
- DxCodes:
- Disease diagnostic codes
- CNS:
- Central nervous samples
- PRRSV:
- Porcine reproductive and respiratory syndrome virus
- IAV:
- Influenza a vírus
References
-
Swine health information center. Swine bacterial disease matrix [internet]. 2021 [cited 2023 Jan 9]. Available from: https://www.swinehealth.org/swine-bacterial-disease-matrix/
-
Hayer SS, Rovira A, Olsen K, Johnson TJ, Vannucci F, Rendahl A, et al. Prevalence and time trend analysis of antimicrobial resistance in respiratory bacterial pathogens collected from diseased pigs in USA between 2006–2016. Res Vet Sci. 2020;128:135–44.
-
Burrough ER, Baum DH, Schwartz KJ. Collecting evidence and establishing causality. In: Diseases of swine. Wiley; 2019. p. 112–22.
-
Aragon V, Segalés J, Tucker AW. Glässer’s Disease. In: Diseases of Swine. Wiley; 2019. p. 844–53.
-
Gottschalk M, Segura M. Streptococcosis. In: Diseases of swine. Wiley; 2019. p. 934–50.
-
Gottschalk M, Broes A. Actinobacillosis. In: Diseases of swine. Wiley; 2019. p. 749–66.
-
Clavijo M, Mugabi R, Ganwu L. Friend or foe: what next generation sequencing can tell you about the endemic agents in your herd. In: Proceedings of the 52nd Annual Meeting of the American Association of Swine Veterinarians. San Francisco; 2021. p. 376–7.
-
Dial G, Rademacher C, Wiseman B, Roker J, Freking B. Costs, consequences and control of endemic diseases. In: 2nd London swine conference. London and Ontario; 2002.
-
Wei YW, Zhu HZ, Huang LP, Xia DL, Wu HL, Bian HQ, et al. Efficacy in pigs of a new inactivated vaccine combining porcine circovirus type 2 and mycoplasma hyorhinis. Vet Microbiol. 2020;242:108588.
-
Obradovic MR, Segura M, Segalés J, Gottschalk M. Review of the speculative role of co-infections in Streptococcus suis-associated diseases in pigs. Vet Res. 2021;52(1):49.
-
Oliveira S, Pijoan C, Morrison R. Evaluation of Haemophilus parasuis control in the nursery using vaccination and controlled exposure. J Swine Health Prod. 2004;12(3):123–8.
-
Giménez-Lirola LG, Meiroz-De-Souza-Almeida H, Magtoto RL, McDaniel AJ, Merodio MM, Matias Ferreyra FS, et al. Early detection and differential serodiagnosis of mycoplasma hyorhinis and mycoplasma hyosynoviae infections under experimental conditions. PLoS One. 2019;14(10):e0223459.
-
Salogni C, Capucchio MT, Colombino E, Pozzi P, Pasquali P, Alborali GL. Bacterial polyarthritis in post-weaning pigs in a high-density swine breeding area in Italy. J Vet Diagn Investig. 2022;34(4):709–11.
-
Miniats O, Spinato M, Sanford S. Actinobacillus suis septicemia in mature swine: two outbreaks resembling erysipelas. Can Vet J. 1989;30(12):943–7.
-
Yaeger MJ. An outbreak of Actinobacillus Suis septicemia in grow/finish pigs. J Vet Diagn Investig. 1996;8(3):381–3.
-
MacInnes J, Gottschalk M, Lone A, Metcalf D, Ojha S, Rosendal T, et al. Prevalence of Actinobacillus pleuropneumoniae, Actinobacillus suis, Haemophilus parasuis, Pasteurella multocida, and Streptococcus suis in representative Ontario swine herds. Can J Vet Res. 2008;72(3):242–8.
-
Hagedorn-Olsen T, Nielsen NC, Friis NF, Nielsen J. Department of Clinical Studies, the Royal Veterinary and Agricultural University, Copenhagen, Denmark. J Veterinary Med Ser A. 1999;46(9):555–64.
-
Nielsen EO, Nielsen NC, Friis NF. Mycoplasma hyosynoviae arthritis in grower-finisher pigs. J Veterinary Med Ser A. 2001;48(8):475–86.
-
Arruda PHE, Gauger P. Optimizing sample selection, collection, and submission to optimize diagnostic value. In: Diseases of Swine. Wiley; 2019. p. 98–111.
-
Derscheid RJ, Rahe MC, Burrough ER, Schwartz KJ, Arruda B. Disease diagnostic coding to facilitate evidence-based medicine: current and future perspectives. J Vet Diagn Investig. 2021;33(3):419–27.
-
Markey B, Leonard F, Archambault M, Cullinane A, Maguire D. Clinical veterinary microbiology. 2nd ed. Mosby Ltd. (UK); 2013. p. 9780702055881.
-
Buchan BW, Ledeboer NA. Emerging Technologies for the Clinical Microbiology Laboratory. Clin Microbiol Rev. 2014;27(4):783–822.
-
Burrough E, Schwartz A, Gauger P, Harmon K, Krull A, Schwartz K. Comparison of postmortem airway swabs and lung tissue for detection of common porcine respiratory pathogens by bacterial culture and polymerase chain reaction assays. J Swine Health Prod. 2018;26(5):246–52.
-
Gomes Neto JC, Bower L, Erickson BZ, Wang C, Raymond M, Strait EL. Quantitative real-time polymerase chain reaction for detecting mycoplasma hyosynoviae and mycoplasma hyorhinis in pen-based oral, tonsillar, and nasal fluids. J Vet Sci. 2015;16(2):195.
-
Trevisan G, Schwartz KJ, Burrough ER, Arruda B, Derscheid RJ, Rahe MC, et al. Visualization and application of disease diagnosis codes for population health management using porcine diseases as a model. J Vet Diagn Investig. 2021;33(3):428–38.
-
Trevisan G, Linhares LCM, Schwartz KJ, Burrough ER, Magalhães E de S, Crim B, et al. Data standardization implementation and applications within and among diagnostic laboratories: integrating and monitoring enteric coronaviruses. J Vet Diagn Investig. 2021 ;33(3):457–468.
-
Trevisan G, Linhares LCM, Crim B, Dubey P, Schwartz KJ, Burrough ER, et al. Macroepidemiological aspects of porcine reproductive and respiratory syndrome virus detection by major United States veterinary diagnostic laboratories over time, age group, and specimen. PLoS One. 2019;14(10):e0223544.
-
USDA. US State Rankings – 2021 Inventory [Internet]. 2022 [cited 2023 Jan 9]. Available from: https://app.usda-reports.penguinlabs.net/?crop=hogs_breeding&statistic=inventory_head&year=2021
-
Rademacher C, Pudenz C, Schulz L. Impact assessment of new US Food and Drug Administration regulations on antibiotic use: a post-enactment survey of swine practitioners. J Swine Health Prod. 2019;27(4):210–20.
-
Lekagul A, Tangcharoensathien V, Yeung S. Patterns of antibiotic use in global pig production: a systematic review. Vet Anim Sci. 2019;7:100058.
-
Clavijo MJ, Sreevatsan S, Johnson TJ, Rovira A. Molecular epidemiology of mycoplasma hyorhinis porcine field isolates in the United States. PLoS One. 2019;14(10):e0223653.
-
Spiegel K, O’Hara K, Vanicek C, Beemer O. United States Department of Agriculture. 2022 [Cited 2023 Jan 9]. Swine Hemorrhagic Fevers: African and Classical Swine Fevers Integrated Surveillance Plan. Available from: https://www.aphis.usda.gov/animal_health/downloads/animal_diseases/swine/hemorrhagic-fevers-integrated-surveillance-plan.pdf.
-
Estrada AA, Gottschalk M, Rossow S, Rendahl A, Gebhart C, Marthaler DG. Serotype and genotype (multilocus sequence type) of Streptococcus suis isolates from the United States serve as predictors of Pathotype. J Clin Microbiol. 2019;57(9):10–1128.
-
Mugabi R, Silva APSP, Hu X, Gottschalk M, Aragon V, Macedo NR, et al. Molecular characterization of Glaesserella parasuis strains circulating in North American swine production systems. BMC Vet Res. 2023;19(1):135. https://doi.org/10.1186/s12917-023-03698-x.
-
Macedo N, Gottschalk M, Strutzberg-Minder K, Van CN, Zhang L, Zou G, et al. Molecular characterization of Glaesserella parasuis strains isolated from North America, Europe and Asia by serotyping PCR and LS-PCR. Vet Res. 2021;52(1):68.
-
Kikuti M, Paploski IAD, Pamornchainavakul N, Picasso-Risso C, Schwartz M, Yeske P, et al. Emergence of a new lineage 1C variant of porcine reproductive and respiratory syndrome virus 2 in the United States. Front Vet Sci. 2021;18:8.
-
Vötsch D, Willenborg M, Weldearegay YB, Valentin-Weigand P. Streptococcus suis – the “two faces” of a Pathobiont in the porcine respiratory tract. Front Microbiol. 2018;15:9.
-
Pan Z, Ma J, Dong W, Song W, Wang K, Lu C, et al. Novel variant serotype of Streptococcus suis isolated from piglets with meningitis. Appl Environ Microbiol. 2015;81(3):976–85.
-
Pan Z, Ma Y, Ma J, Dong W, Yao H. Acute meningitis of piglets and mice caused by co-infected with Streptococcus suis and Aerococcus viridans. Microb Pathog. 2017;106:60–4.
-
Ni HB, Gong QL, Zhao Q, Li XY, Zhang XX. Prevalence of Haemophilus parasuis“Glaesserella parasuis” in pigs in China: a systematic review and meta-analysis. Prev Vet Med. 2020;182:105083.
-
Palzer A, Haedke K, Heinritzi K, Zoels S, Ladinig A, Ritzmann M. Associations among Haemophilus parasuis, mycoplasma hyorhinis, and porcine reproductive and respiratory syndrome virus infections in pigs with polyserositis. Can Vet J. 2015;56(3):285–7.
-
Palzer A, Ritzmann M, Hafner-Marx A, Wolf G, Heinritzi K. Detection of Haemophilus parasuis and mycoplasma hyorhinis in swine and association of those pathogens with clinical and pathological-anatomic findings. Dtsch Tierarztl Wochenschr. 2006;113(6):227–30.
-
Lee JA, Oh YR, Hwang MA, Lee JB, Park SY, Song CS, et al. Mycoplasma hyorhinis is a potential pathogen of porcine respiratory disease complex that aggravates pneumonia caused by porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2016;177:48–51.
-
Merodio M, Groeltz J, Piñeyro P, Derscheid R. Retrospective analysis of Mycoplasma hyorhinis pulmonary and systemic infection in diagnostic cases with correlation of qPCR Ct values and detection by RNAscope®. In: In: 53rd Annual Meeting of the American Association of Swine Veterinarians. Indiannapolis IN USA; 2022.
-
Clavijo MJ, Murray D, Oliveira S, Rovira A. Infection dynamics of mycoplasma hyorhinis in three commercial pig populations. Vet Rec. 2017;181(3):68–8.
-
Van Ostaaijen J, Frey J, Rosendal S, MacInnes JI. Actinobacillus suis strains isolated from healthy and diseased swine are clonal and carry apxICABDvar. Suis and apxIICAvar. Suis toxin genes. J Clin Microbiol. 1997;35(5):1131–7.
-
Ojha S, Lacouture S, Gottschalk M, MacInnes JI. Characterization of colonization-deficient mutants of Actinobacillus suis. Vet Microbiol. 2010;140(1–2):122–30.
-
Mahan-Riggs E. Three cases of Actinobacillus suis in eastern North Carolina. J Swine Health Prod. 2022;30(1):24–30.
-
Roos LR, Surendran Nair M, Rendahl AK, Pieters M. Mycoplasma hyorhinis and mycoplasma hyosynoviae dual detection patterns in dams and piglets. PLoS One. 2019;14(1):e0209975.
-
Pillman D, Surendran Nair M, Schwartz J, Pieters M. Detection of mycoplasma hyorhinis and mycoplasma hyosynoviae in oral fluids and correlation with pig lameness scores. Vet Microbiol. 2019;239:108448.
-
Hallowell A, Pierdon M. Effects of lameness on productivity and longevity for sows in pen gestation. J Swine Health Prod. 2022;30(4):223–9.
-
Heinonen M, Peltoniemi O, Valros A. Impact of lameness and claw lesions in sows on welfare, health and production. Livest Sci. 2013;1(156):2–9.
-
Canning P, Costello N, Mahan-Riggs E, Schwartz K, Skoland K, Crim B, et al. Retrospective study of lameness cases in growing pigs associated with joint and leg submissions to a veterinary diagnostic laboratory. J Swine Health Prod. 2019;27(3):118–24. Available from: https://www.aasv.org/shap/issues/v27n3/v27n3p118.pdf
-
Brockmeier SL, Halbur PG, Thacker EL. Porcine respiratory disease complex. In: Polymicrobial Diseases. Washington, DC, USA: ASM Press; 2014. p. 231–58.
-
Sarli G, D’Annunzio G, Gobbo F, Benazzi C, Ostanello F. The role of pathology in the diagnosis of swine respiratory disease. Vet Sci. 2021;8(11):256.
-
Siqueira FM, Pérez-Wohlfeil E, Carvalho FM, Trelles O, Schrank IS, Vasconcelos ATR, et al. Microbiome overview in swine lungs. PLoS One. 2017;12(7):e0181503.
-
Santos J, Schwartz K, Derscheid R, Magstadt D, Burrough E, Gauger P, et al. Distribution and characterization of Streptococcus suis strains of clinical importance within the US swine herd. 2022. https://doi.org/10.54846/am2022/110.