

Key takeaways
Lincomix is the only Type A medicated feed with a Mycoplasma hyopneumoniae (Mhp) label claim.*
Lincomix is approved to control Lawsonia intracellularis at the same rate as the minimum dose for Mhp allowing for two diseases to be addressed at once.
In a study, when Lincomix was fed at 100 grams per ton for 21 days, pigs consuming Lincomix had reduced lung lesions and coughing scores at 21 days compared to controls.1
*Lincomix is approved for the reduction in severity of effects of respiratory disease associated with Mhp.
The feed medication Lincomix® (lincomycin hydrochloride) has been used for decades to reduce the severity of pneumonia in pigs caused by Mhp. Can you tell us more about this product?
Lincomix can be used at any rate between 100 and 200 grams per ton for 21 days for reduction in the severity of the effects of respiratory disease associated with Mhp. This gives veterinarians flexibility in dosing.
In addition, Lincomix is approved for controlling swine dysentery and ileitis due to Lawsonia intracellularis at 40 grams per ton. It is also approved for treating swine dysentery and controlling ileitis at 100 grams per ton. When used at the 100 grams per ton level, it should be fed for 21 days or until signs of clinical disease disappear.
Because the Lincomix dose rate for swine dysentery and ileitis overlap with the minimum dose rate for Mhp, one product can be used to address two diseases when needed at the 100 grams per ton level.
What is the withdrawal time for Lincomix?
There is a zero-day withdrawal period for Lincomix, making it a convenient choice even in late finishing disease breaks.
How effective is the 100 grams per ton Lincomix dosage for Mhp?
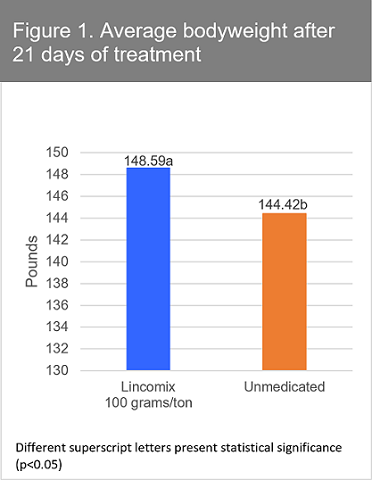
Zoetis conducted a study involving more than 1,700 pigs at eight US swine operations. Across all sites, 28-76% of pigs were positive for Mhp. When Lincomix was fed at 100 grams per ton for 21 days, pigs consuming Lincomix had reduced lung lesions and coughing scores at 21 days compared to controls. The Lincomix groups also demonstrated better average daily gain, feed efficiency and final bodyweight compared to untreated controls. The differences were significant (p < 0.05). Treated pigs were nearly 4.2 pounds heavier than non-medicated control pigs at the end of the 21-day period (Figure 1). 1

Mhp has been around a long time. Why is it still a problem?
It’s estimated Mhp is present in more than 50% of U.S. herds.2 It remains a top cause of pneumonia in the presence of other bacteria and viruses.3 It can occur in young pigs but usually affects finishing-age pigs, when producers have already made a considerable investment in those animals.
Mhp is a tough organism to control because the organism targets the respiratory tract cilia and can persist for weeks. Some pigs don’t show signs of infection, but can still infect other pigs. Mhp not only causes poor feed conversion and growth, but it also increases susceptibility to other respiratory infections, and is a component of porcine respiratory disease complex.4,5
What else can be done to improve Mhp control?
You need to take an integrated, holistic approach using other tools available in addition to in-feed medication use. Vaccination can help reduce Mhp shedding in the nursery and potentially reduce vertical and horizontal Mhp transmission.2 Some veterinarians advise administration of a long-acting injectable antimicrobial to young pigs. In addition, the introduction of replacement gilts must be carefully managed — and maintaining good biosecurity is imperative.
Another consideration is whether elimination of Mhp is feasible for your operation. To learn more on this topic, CLICK HERE.
How can Zoetis help producers achieve optimal results with Lincomix?
There are a couple of different ways we do this. Through our STOMP PLUS® diagnostics program, we first seek to understand when Mhp seroconversion is occurring by collecting blood samples from pigs across different ages (a cross-sectional study). Knowing when most pigs are becoming infected with Mhp allows for optimal Lincomix timing. Lincomix should generally be placed 5-6 weeks before seroconversion.
Once Lincomix is implemented, we also can validate inclusion levels of Lincomix in feed samples to further promote responsible antibiotic use. This is done through our Customer Analytical Support Laboratory (CASL). Your Zoetis technical services veterinarian is equipped to help with both of these to ensure producers achieve optimal results when using Lincomix.
Caution: Federal law restricts medicated feed containing this veterinary feed directive (VFD) drug to use by or on the order of a licensed veterinarian.
Do not use LINCOMIX in swine intended for breeding. Do not allow unapproved species access to feeds containing lincomycin.
References
- Data on file, Study Report No. A121C-US-14-150, Zoetis Inc.
- Thacker EL, Minion FC. Mycoplasmosis. In:Zimmerman JJ, Karriker LA, Schwartz KJ, et al, eds. Diseases of Swine, 10th ed. Oxford, UK:Wiley-Blackwell; 2012:779-797.
- Thacker EL, et al. Mycoplasmal Pneumonia of Swine, Cooperative Extension, 2010.
- Mycoplasma Pneumonia (Enzootic Pneumonia), Iowa State University of Science and Technology, College of Veterinary Medicine. 2017 Feb 4.
- Maes, D, et al. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control. Transbound Emerg Dis. 2018 May;65(S1):110-124.







