
Key takeaways
To understand how quickly EXCEDE® for Swine (CCFA) reaches concentrations at or above MIC90 levels for Streptococcus suis specifically, the objective of this study was to uncover the pharmacokinetics of CCFA in the first hour after a single intramuscular dose.
Within 15 minutes of administration, pigs achieved levels greater than five times the minimum inhibitory concentration (MIC) needed to prevent growth of nearly 100% of target respiratory pathogens.
For Streptococous suis, EXCEDE® for Swine reached therapeutic plasma concentrations in all pigs within 30 minutes post-injection.
Swine practitioners consider many factors when implementing antibiotic regimens, including labeled pathogens, clinical breakpoints or minimum inhibitory concentrations (MIC), cost, ease of implementation, and withdrawal times. Duration of therapy is another factor to consider, especially with today’s challenges in labor.
EXCEDE® for Swine (ceftiofur crystalline free acid) has demonstrated a duration of therapy of seven days in an APP challenge model, reaching plasma concentrations of 2.23 ug/mL at one-hour post-injection. This is above the MIC90 for Streptococcus suis (2.0 ug/mL) and for Actinobacillus pleuropneumoniae (APP), Pasteurella multocida (PM), and Haemophilus [Glaesserella] parasuis (HPS/GPS) (all <0.03 ug/mL). However, little is known about the pharmacokinetics of CCFA at shorter time periods post-injection, specifically in the first hour.
Sarah Lutz, a third-year veterinary student at Auburn University, interned with Zoetis in 2023 as part of the Swine Veterinary Internship Program (SVIP)Opens in a new window. During her internship, she collaborated with Doug Powers, DVM with Four Star Veterinary Service, on a study to understand how quickly EXCEDE® for Swine reaches therapeutic concentrations.
Pharmacokinetics in the first hour
“We wanted to know more about the pharmacokinetics of EXCEDE® for Swine,” said Lutz. “Previously published clinical data showed that EXCEDE® for Swine has a long duration of therapy, but the first time point measured was at one-hour post-injection. The objective of my study was to look at the pharmacokinetics in the first hour after administration of EXCEDE® for Swine.”
Six 21-day old pigs of mixed sex were selected, weighed, and enrolled from two litters. A labeled dose of 1 ml/44 lb (5.0 mg/kg) of EXCEDE® for Swine was calculated and administered to each pig.1
“We collected blood immediately prior to an intramuscular dose of EXCEDE® for Swine,” she explained. “Then we measured time points at 5, 10, 15, 30, 45 and 60 minutes and used a derivatization method and reverse phase high pressure liquid chromatography to measure the concentration of the ceftiofur and its active metabolite, desfuroylceftiofur (DFC).”
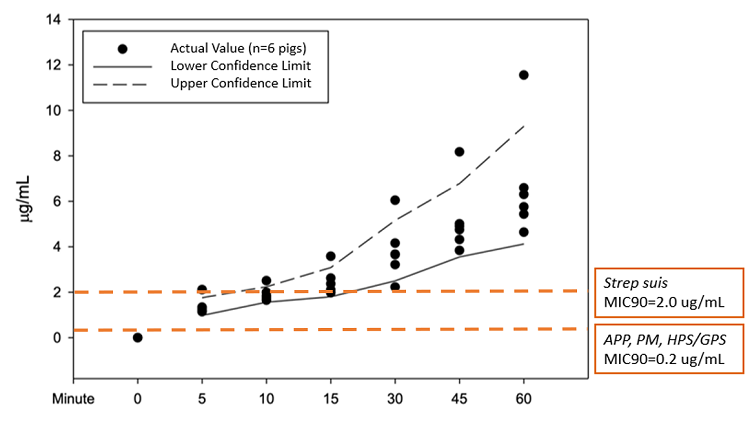
The results at 15 minutes showed five out of six pigs reached therapeutic plasma concentration levels greater than the MIC90 of S. suis (Figure 1) At 30 minutes, all six pigs reached plasma concentrations above the MIC90 for S. suis. For APP and P. multocida, MIC90 is lower and CCFA reached therapeutic plasma concentrations within 5 minutes post-injection for all six pigs.
Mean plasma concentrations by time were:
- 1.37 ug/ml at 5 minutes
- 1.90 ug/ml at 10 minutes
- 2.44 ug/ml at 15 minutes
- 3.83 ug/ml at 30 minutes
- 5.16 ug/ml at 45 minutes
- 6.71 ug/ml at 60 minutes
Figure 1: Ceftiofur and desfuroylceftiofur plasma concentrations post-injection
Benefit for producers
“Producers and practitioners can be confident that EXCEDE® for Swine has not only a long duration of therapy, but it also reaches therapeutic plasma levels quickly,” said Lutz. “This was a little faster than expected, which is really exciting.”
Important Safety Information
People with known hypersensitivity to penicillin or cephalosporins should avoid exposure to EXCEDE® for Swine. Do not use in swine found to be hypersensitive to the product. Pre-slaughter withdrawal time is 14 days following the last dose. See full prescribing information at excedeforswine.com/pi.
References
- Lutz S, Jansen M, and Cox S. Pharmacokinetics of Ceftiofur Crystalline Free Acid. Proceedings Leman Swine Conference, St. Paul 2023.





