
Key takeaways
Pigs administered Draxxin® at weaning demonstrated consistent performance improvement in the face of complex swine respiratory disease.
The administration of Draxxin to pigs at weaning resulted in reduced mortality and fewer retreats, pulls, and lightweight culls.
Pigs administered Draxxin at weaning showed a 1.54 – 2.91 lb weight advantage1-3 through the nursery and a 3.23 – 4.2 lb weight advantage through the finisher.1-2,4
Swine respiratory disease (SRD) is the leading cause of swine mortality in the United States.5,6 When both bacterial and viral pathogens are involved, we often call this complex SRD. Zoetis is committed to investing in and discovering new approaches in controlling complex SRD. With this goal in mind, Zoetis collaborated on four trials during the weaning phase to learn more.
“Weaning is a time of immense stress for pigs, making it the perfect time to study and learn more about complex SRD,” said Dr. Eva Jablonski. “Continuing to discover and test new approaches to controlling SRD is important. If we can better understand Draxxin’s role, it puts us in a better position to help producers and veterinarians control complex SRD, especially during the post-weaning phase.”
Study design
The four studies were conducted in the Midwest with a total of 6,253 weaned pigs. Three studies were held under commercial field conditions, and one was conducted at a contract research facility. Each farm was experiencing underlying viral activity, i.e. complex SRD with varying prevalence of porcine reproductive and respiratory syndrome virus (PRRSv).
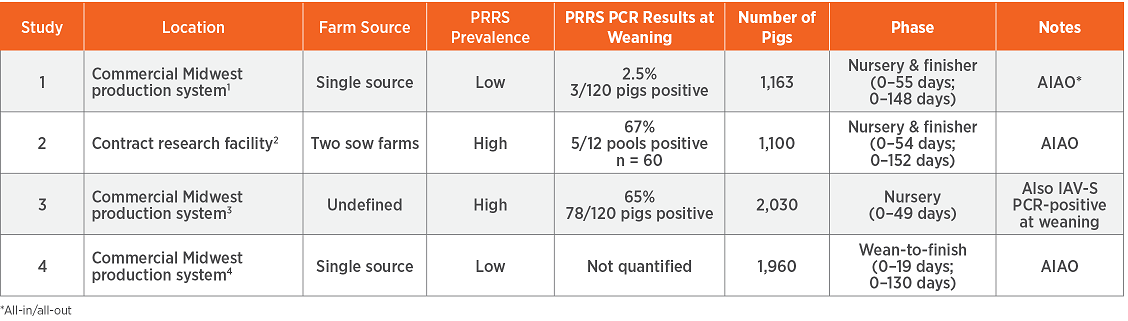
Draxxin or saline was administered at weaning for SRD control, and the pigs were commingled after weaning. Each pig was weighed at weaning and at the end of the nursery phase; in three studies, pigs were also weighed at the end of the finishing phase. The caregivers observing the weaned pigs were blinded to treatment. The studies measured several variables including mortality, average daily gain (ADG), nursery and finishing weights, retreatments in three studies and pulls and lightweight culls in two studies.
Study summary results
All studies showed a reduction in mortality in Draxxin-treated pigs. Three studies (1, 2, and 3) showed a statistically significant reduction in mortality. Pigs treated with Draxxin, resulting in a 22% – 54% reduction in mortality.
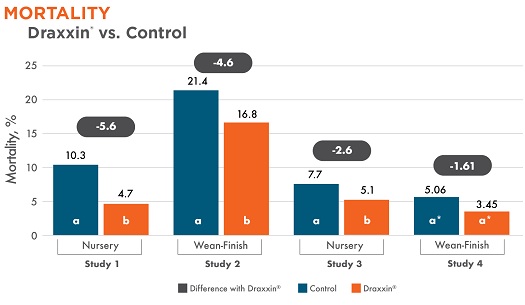
Different letters (a, b) indicate statistically significant difference (P < 0.05) between columns. *Study 4 trending toward significance with P-value = 0.0791.
Three studies measured retreatment rates. There was a statistically significant difference with fewer Draxxin-treated pigs needing retreatment in two studies (2 and 3).
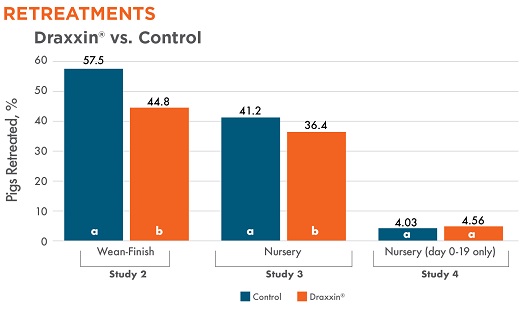
Pulls to hospital pen were not documented in Study 1. Different letters (a, b) indicate statistically significant difference (P < 0.05) between columns.
Two studies measured sick pigs that required pulling after their initial treatment. There was a statistically significant difference with fewer Draxxin-treated pigs pulled to a hospital pen in both studies (2 and 3).
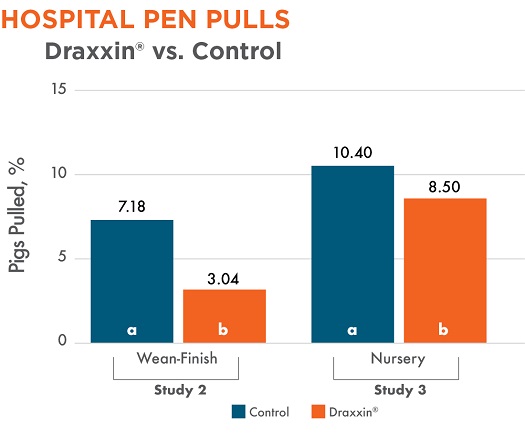
Different letters (a, b) indicate statistically significant difference (P < 0.05) between columns.
There was no significant difference in weaning weight across treatment groups in three of the studies (1, 3 and 4). There was a statistically significant difference in ending nursery weight in all studies. Pigs treated with Draxxin had a 1.54 – 2.91 lb weight advantage through the nursery period (studies 1, 2 and 3).
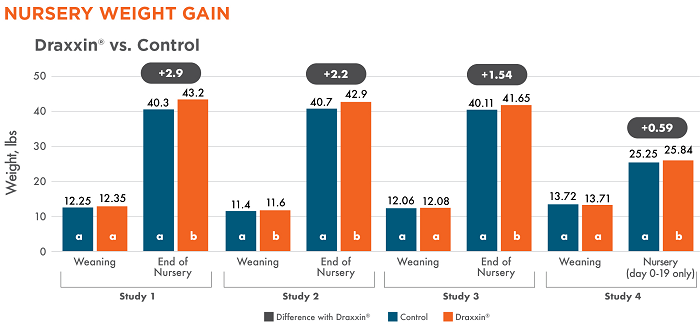
Different letters (a, b) indicate statistically significant difference (P < 0.05) between columns.
Three studies continued to measure weight gain after the nursery period through finishing. The significant weight differences at the end of the nursery period were maintained through the finishing period. Pigs treated with Draxxin had a 3.23 – 4.2 lb weight advantage through the finishing period (studies 1, 2 and 4).
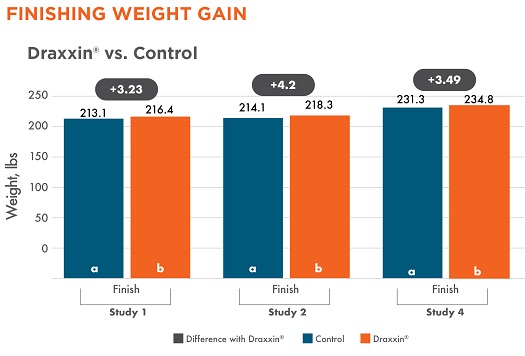
Different letters (a, b) indicate statistically significant difference (P < 0.05) between columns.
All four studies measured ADG through nursery; three also measured ADG though finishing. All four studies showed a statistically significant difference with Draxxin-treated pigs having a significantly higher ADG through the nursery period ranging from 0.03 – 0.06 lb/day. Two studies showed a statistically significant difference with Draxxin-treated pigs having a significantly higher ADG through the finisher period (studies 2 and 4) of ~0.03 lb/day.
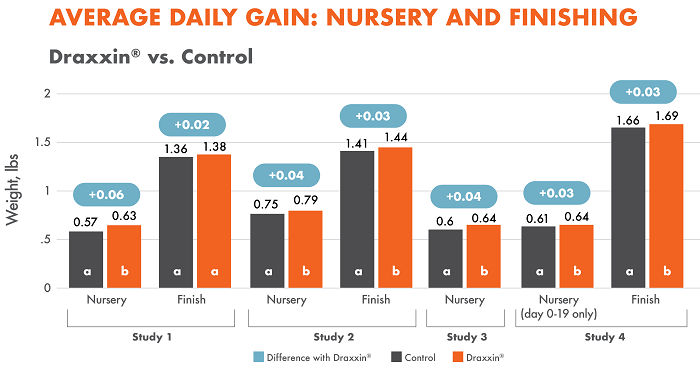
Different letters (a, b) indicate statistically significant difference (P < 0.05) between columns.
Two studies tracked cull and lightweight pigs through marketing (studies 2 and 4). Both studies showed numerically fewer cull and lightweight pigs when treated with Draxxin, ranging from 0.09% – 3.45%.
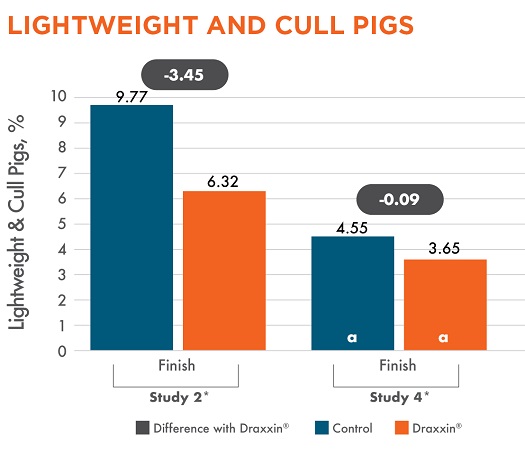
Different letters (a, b) indicate statistically significant difference (P < 0.05) between columns. *Study 2 target weight = 180 lbs; Study 4 target weight = 180 lbs
Conclusions
Draxxin® administration at weaning helped provide consistent improvement in pig performance in the face of complex SRD with underlying viral activity. The studies showed administration of Draxxin at weaning resulted in reduced mortality, improved ADG, higher nursery and finishing weights and fewer retreats, pulls and lightweight culls.
- 22% – 54% reduction in mortality through nursery1,3 and wean-to-finish2,4
- Statistically fewer pigs needing retreatment2-3
- Statistically fewer pigs pulled to hospital pen2-3
- 1.54 – 2.91-lb weight advantage through nursery1-3
- 3.23 – 4.2-lb weight advantage through finisher1-2,4
“These studies illustrate improvements in pig performance from Draxxin use at weaning, which subsequently means increased economic returns for our producers,” said Dr. Jablonski. “When we know a mix of SRD pathogens are involved, regardless of the prevalence level, Draxxin provides effective extended therapy for swine respiratory disease that shows results when it really counts – in improved post-weaning performance.”
IMPORTANT SAFETY INFORMATION: Withdraw DRAXXIN/DRAXXIN 25 five (5) days prior to slaughter in swine. Do not use in animals known to be hypersensitive to the product. See full Prescribing Information at www.draxxinpork.com/pi or www.draxxin25.com/pi.





